Plus Sized Fashion Design Model Sketch
Drug pattern, often referred to as rational drug design or simply rational design, is the inventive process of finding new medications based on the cognition of a biological target.[1] The drug is almost commonly an organic small molecule that activates or inhibits the function of a biomolecule such as a poly peptide, which in plow results in a therapeutic do good to the patient. In the most basic sense, drug blueprint involves the blueprint of molecules that are complementary in shape and charge to the biomolecular target with which they collaborate and therefore will bind to it. Drug design oftentimes simply non necessarily relies on estimator modeling techniques.[2] This type of modeling is sometimes referred to as computer-aided drug design. Finally, drug blueprint that relies on the knowledge of the three-dimensional structure of the biomolecular target is known every bit structure-based drug design.[two] In add-on to small molecules, biopharmaceuticals including peptides[3] [4] and especially therapeutic antibodies are an increasingly important class of drugs and computational methods for improving the analogousness, selectivity, and stability of these protein-based therapeutics have too been developed.[5]
The phrase "drug design" is to some extent a misnomer. A more accurate term is ligand design (i.e., design of a molecule that volition bind tightly to its target).[half dozen] Although blueprint techniques for prediction of binding affinity are reasonably successful, in that location are many other properties, such as bioavailability, metabolic half-life, side effects, etc., that kickoff must be optimized before a ligand can become a safe and efficacious drug. These other characteristics are often difficult to predict with rational pattern techniques. Nevertheless, due to high attrition rates, specially during clinical phases of drug development, more attention is being focused early in the drug design process on selecting candidate drugs whose physicochemical properties are predicted to result in fewer complications during development and hence more than likely to lead to an canonical, marketed drug.[7] Furthermore, in vitro experiments complemented with computation methods are increasingly used in early drug discovery to select compounds with more favorable ADME (absorption, distribution, metabolism, and excretion) and toxicological profiles.[8]
Drug targets [edit]
A biomolecular target (most commonly a protein or a nucleic acrid) is a key molecule involved in a particular metabolic or signaling pathway that is associated with a specific disease condition or pathology or to the infectivity or survival of a microbial pathogen. Potential drug targets are not necessarily disease causing but must by definition exist illness modifying.[9] In some cases, pocket-sized molecules will be designed to heighten or inhibit the target role in the specific affliction modifying pathway. Small molecules (for example receptor agonists, antagonists, inverse agonists, or modulators; enzyme activators or inhibitors; or ion channel openers or blockers)[10] will exist designed that are complementary to the bounden site of target.[11] Pocket-size molecules (drugs) can be designed so every bit non to touch any other important "off-target" molecules (often referred to every bit antitargets) since drug interactions with off-target molecules may lead to undesirable side effects.[12] Due to similarities in bounden sites, closely related targets identified through sequence homology have the highest risk of cantankerous reactivity and hence highest side effect potential.
Most commonly, drugs are organic modest molecules produced through chemical synthesis, only biopolymer-based drugs (also known every bit biopharmaceuticals) produced through biological processes are becoming increasingly more common.[xiii] In addition, mRNA-based gene silencing technologies may have therapeutic applications.[14]
Rational drug discovery [edit]
In contrast to traditional methods of drug discovery (known every bit forrad pharmacology), which rely on trial-and-error testing of chemical substances on cultured cells or animals, and matching the apparent effects to treatments, rational drug design (also called opposite pharmacology) begins with a hypothesis that modulation of a specific biological target may have therapeutic value. In guild for a biomolecule to be selected as a drug target, two essential pieces of information are required. The first is show that modulation of the target will be illness modifying. This knowledge may come from, for instance, disease linkage studies that show an association between mutations in the biological target and certain disease states.[15] The second is that the target is "druggable". This means that it is capable of bounden to a pocket-size molecule and that its activity can be modulated past the small molecule.[xvi]
In one case a suitable target has been identified, the target is ordinarily cloned and produced and purified. The purified poly peptide is so used to establish a screening assay. In addition, the three-dimensional structure of the target may be determined.
The search for small molecules that bind to the target is begun past screening libraries of potential drug compounds. This may be done by using the screening analysis (a "moisture screen"). In improver, if the structure of the target is available, a virtual screen may exist performed of candidate drugs. Ideally the candidate drug compounds should be "drug-like", that is they should possess properties that are predicted to lead to oral bioavailability, adequate chemic and metabolic stability, and minimal toxic effects.[17] Several methods are available to estimate druglikeness such as Lipinski'south Rule of V and a range of scoring methods such as lipophilic efficiency.[18] Several methods for predicting drug metabolism have too been proposed in the scientific literature.[19]
Due to the large number of drug properties that must exist simultaneously optimized during the design process, multi-objective optimization techniques are sometimes employed.[twenty] Finally because of the limitations in the current methods for prediction of action, drug design is all the same very much reliant on serendipity[21] and bounded rationality.[22]
Computer-aided drug design [edit]
The most cardinal goal in drug design is to predict whether a given molecule will demark to a target and if and then how strongly. Molecular mechanics or molecular dynamics is about often used to gauge the strength of the intermolecular interaction between the minor molecule and its biological target. These methods are also used to predict the conformation of the small-scale molecule and to model conformational changes in the target that may occur when the pocket-sized molecule binds to it.[3] [4] Semi-empirical, ab initio quantum chemical science methods, or density functional theory are oft used to provide optimized parameters for the molecular mechanics calculations and too provide an estimate of the electronic properties (electrostatic potential, polarizability, etc.) of the drug candidate that will influence binding affinity.[23]
Molecular mechanics methods may too be used to provide semi-quantitative prediction of the bounden affinity. Also, knowledge-based scoring function may be used to provide bounden analogousness estimates. These methods employ linear regression, machine learning, neural nets or other statistical techniques to derive predictive bounden affinity equations by fitting experimental affinities to computationally derived interaction energies between the modest molecule and the target.[24] [25]
Ideally, the computational method volition be able to predict affinity before a compound is synthesized and hence in theory but one compound needs to be synthesized, saving enormous time and price. The reality is that present computational methods are imperfect and provide, at best, only qualitatively accurate estimates of analogousness. In practise it still takes several iterations of pattern, synthesis, and testing before an optimal drug is discovered. Computational methods have accelerated discovery by reducing the number of iterations required and have often provided novel structures.[26] [27]
Drug design with the assist of computers may exist used at any of the following stages of drug discovery:
- hit identification using virtual screening (construction- or ligand-based design)
- striking-to-pb optimization of affinity and selectivity (structure-based design, QSAR, etc.)
- lead optimization of other pharmaceutical properties while maintaining affinity

Flowchart of a Usual Clustering Analysis for Structure-Based Drug Design
In social club to overcome the insufficient prediction of bounden affinity calculated by recent scoring functions, the protein-ligand interaction and compound 3D structure data are used for assay. For structure-based drug design, several mail-screening analyses focusing on protein-ligand interaction have been developed for improving enrichment and finer mining potential candidates:
- Consensus scoring[28] [29]
- Selecting candidates by voting of multiple scoring functions
- May lose the human relationship between poly peptide-ligand structural information and scoring criterion
- Cluster analysis[30] [31]
- Represent and cluster candidates according to poly peptide-ligand 3D information
- Needs meaningful representation of protein-ligand interactions.
Types [edit]
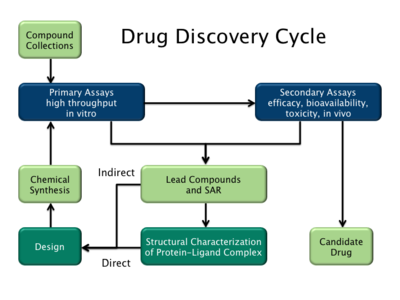
Drug discovery cycle highlighting both ligand-based (indirect) and structure-based (direct) drug blueprint strategies.
There are two major types of drug pattern. The starting time is referred to as ligand-based drug pattern and the 2nd, construction-based drug design.[2]
Ligand-based [edit]
Ligand-based drug design (or indirect drug pattern) relies on noesis of other molecules that bind to the biological target of interest. These other molecules may be used to derive a pharmacophore model that defines the minimum necessary structural characteristics a molecule must possess in lodge to bind to the target.[32] In other words, a model of the biological target may be built based on the cognition of what binds to it, and this model in turn may exist used to design new molecular entities that collaborate with the target. Alternatively, a quantitative structure-activity relationship (QSAR), in which a correlation betwixt calculated properties of molecules and their experimentally determined biological activity, may be derived. These QSAR relationships in turn may be used to predict the activity of new analogs.[33]
Construction-based [edit]
Structure-based drug design (or straight drug blueprint) relies on knowledge of the 3 dimensional structure of the biological target obtained through methods such as x-ray crystallography or NMR spectroscopy.[34] If an experimental structure of a target is not bachelor, information technology may be possible to create a homology model of the target based on the experimental construction of a related poly peptide. Using the structure of the biological target, candidate drugs that are predicted to bind with high affinity and selectivity to the target may be designed using interactive graphics and the intuition of a medicinal chemist. Alternatively various automated computational procedures may be used to suggest new drug candidates.[35]
Current methods for structure-based drug pattern can be divided roughly into three main categories.[36] The first method is identification of new ligands for a given receptor by searching large databases of 3D structures of small-scale molecules to find those plumbing fixtures the binding pocket of the receptor using fast guess docking programs. This method is known as virtual screening. A second category is de novo design of new ligands. In this method, ligand molecules are built upward within the constraints of the bounden pocket past assembling small pieces in a stepwise manner. These pieces tin be either individual atoms or molecular fragments. The cardinal advantage of such a method is that novel structures, not contained in whatever database, can be suggested.[37] [38] [39] A tertiary method is the optimization of known ligands by evaluating proposed analogs within the binding cavity.[36]
Binding site identification [edit]
Binding site identification is the get-go step in construction based design.[xvi] [40] If the structure of the target or a sufficiently similar homolog is determined in the presence of a leap ligand, and then the ligand should be observable in the structure in which example location of the binding site is trivial. However, there may exist unoccupied allosteric binding sites that may be of interest. Furthermore, information technology may be that simply apoprotein (poly peptide without ligand) structures are available and the reliable identification of unoccupied sites that accept the potential to bind ligands with high affinity is not-trivial. In brief, binding site identification usually relies on identification of concave surfaces on the protein that can accommodate drug sized molecules that also possess appropriate "hot spots" (hydrophobic surfaces, hydrogen bonding sites, etc.) that bulldoze ligand bounden.[16] [40]
Scoring functions [edit]
Structure-based drug design attempts to apply the structure of proteins as a basis for designing new ligands by applying the principles of molecular recognition. Selective high analogousness binding to the target is generally desirable since it leads to more efficacious drugs with fewer side furnishings. Thus, one of the virtually important principles for designing or obtaining potential new ligands is to predict the binding affinity of a sure ligand to its target (and known antitargets) and utilize the predicted analogousness as a criterion for selection.[41]
One early full general-purposed empirical scoring role to draw the binding energy of ligands to receptors was adult past Böhm.[42] [43] This empirical scoring part took the grade:
where:
- ΔG0 – empirically derived kickoff that in office corresponds to the overall loss of translational and rotational entropy of the ligand upon binding.
- ΔGhb – contribution from hydrogen bonding
- ΔGionic – contribution from ionic interactions
- ΔGlip – contribution from lipophilic interactions where |Alipo| is surface area of lipophilic contact betwixt the ligand and receptor
- ΔGrot – entropy penalty due to freezing a rotatable in the ligand bond upon binding
A more than general thermodynamic "master" equation is as follows:[44]
where:
- desolvation – enthalpic penalty for removing the ligand from solvent
- motion – entropic penalisation for reducing the degrees of liberty when a ligand binds to its receptor
- configuration – conformational strain energy required to put the ligand in its "agile" conformation
- interaction – enthalpic gain for "resolvating" the ligand with its receptor
The basic idea is that the overall binding gratuitous energy tin can be decomposed into independent components that are known to exist important for the bounden process. Each component reflects a certain kind of costless energy alteration during the binding process betwixt a ligand and its target receptor. The Master Equation is the linear combination of these components. According to Gibbs free energy equation, the relation between dissociation equilibrium abiding, Kd, and the components of costless energy was built.
Various computational methods are used to estimate each of the components of the primary equation. For example, the change in polar surface surface area upon ligand binding tin be used to estimate the desolvation free energy. The number of rotatable bonds frozen upon ligand binding is proportional to the motion term. The configurational or strain energy can be estimated using molecular mechanics calculations. Finally the interaction free energy tin can be estimated using methods such as the change in non polar surface, statistically derived potentials of mean force, the number of hydrogen bonds formed, etc. In practice, the components of the master equation are fit to experimental data using multiple linear regression. This can be done with a diverse training set including many types of ligands and receptors to produce a less accurate but more than general "global" model or a more restricted set of ligands and receptors to produce a more accurate but less general "local" model.[45]
Examples [edit]
A particular example of rational drug design involves the use of three-dimensional information about biomolecules obtained from such techniques every bit X-ray crystallography and NMR spectroscopy. Computer-aided drug design in particular becomes much more than tractable when in that location is a high-resolution structure of a target poly peptide leap to a stiff ligand. This approach to drug discovery is sometimes referred to as structure-based drug design. The outset unequivocal example of the application of construction-based drug design leading to an canonical drug is the carbonic anhydrase inhibitor dorzolamide, which was approved in 1995.[46] [47]
Some other important case study in rational drug pattern is imatinib, a tyrosine kinase inhibitor designed specifically for the bcr-abl fusion protein that is characteristic for Philadelphia chromosome-positive leukemias (chronic myelogenous leukemia and occasionally acute lymphocytic leukemia). Imatinib is essentially different from previous drugs for cancer, as virtually agents of chemotherapy but target rapidly dividing cells, non differentiating between cancer cells and other tissues.[48]
Additional examples include:
- Many of the atypical antipsychotics
- Cimetidine, the prototypical H2-receptor antagonist from which the later members of the class were developed
- Selective COX-2 inhibitor NSAIDs
- Enfuvirtide, a peptide HIV entry inhibitor
- Nonbenzodiazepines like zolpidem and zopiclone
- Raltegravir, an HIV integrase inhibitor[49]
- SSRIs (selective serotonin reuptake inhibitors), a class of antidepressants
- Zanamivir, an antiviral drug
Case studies [edit]
- v-HT3 antagonists
- Acetylcholine receptor agonists
- Angiotensin receptor antagonists
- Bcr-Abl tyrosine-kinase inhibitors
- Cannabinoid receptor antagonists
- CCR5 receptor antagonists
- Cyclooxygenase ii inhibitors
- Dipeptidyl peptidase-4 inhibitors
- HIV protease inhibitors
- NK1 receptor antagonists
- Non-nucleoside contrary transcriptase inhibitors
- Nucleoside and nucleotide reverse transcriptase inhibitors
- PDE5 inhibitors
- Proton pump inhibitors
- Renin inhibitors
- Triptans
- TRPV1 antagonists
- c-Met inhibitors
Criticism [edit]
It has been argued that the highly rigid and focused nature of rational drug design suppresses serendipity in drug discovery.[50]
Encounter also [edit]
- Bioisostere
- Bioinformatics
- Cheminformatics
- Drug evolution
- Drug discovery
- List of pharmaceutical companies
- Medicinal chemistry
- Molecular blueprint software
- Molecular modification
- Retrometabolic drug pattern
References [edit]
- ^ Madsen U, Krogsgaard-Larsen P, Liljefors T (2002). Textbook of Drug Blueprint and Discovery. Washington, D.C.: Taylor & Francis. ISBN978-0-415-28288-8.
- ^ a b c Reynolds CH, Merz KM, Ringe D, eds. (2010). Drug Design: Construction- and Ligand-Based Approaches (ane ed.). Cambridge, United kingdom of great britain and northern ireland: Cambridge University Press. ISBN978-0521887236.
- ^ a b Fosgerau Chiliad, Hoffmann T (January 2015). "Peptide therapeutics: current status and future directions". Drug Discovery Today. 20 (1): 122–128. doi:10.1016/j.drudis.2014.10.003. PMID 25450771.
- ^ a b Ciemny Thou, Kurcinski Thousand, Kamel K, Kolinski A, Alam Northward, Schueler-Furman O, Kmiecik S (Baronial 2018). "Protein-peptide docking: opportunities and challenges". Drug Discovery Today. 23 (8): 1530–1537. doi:ten.1016/j.drudis.2018.05.006. PMID 29733895.
- ^ Shirai H, Prades C, Vita R, Marcatili P, Popovic B, Xu J, et al. (Nov 2014). "Antibody computer science for drug discovery". Biochimica et Biophysica Acta. 1844 (xi): 2002–2015. doi:10.1016/j.bbapap.2014.07.006. PMID 25110827.
- ^ Tollenaere JP (April 1996). "The role of structure-based ligand design and molecular modelling in drug discovery". Pharmacy World & Science. xviii (2): 56–62. doi:10.1007/BF00579706. PMID 8739258. S2CID 21550508.
- ^ Waring MJ, Arrowsmith J, Leach AR, Leeson PD, Mandrell South, Owen RM, Pairaudeau K, Pennie WD, Pickett SD, Wang J, Wallace O, Weir A (2015). "An analysis of the attrition of drug candidates from 4 major pharmaceutical companies". Nature Reviews Drug Discovery. 14 (seven): 475–86. doi:10.1038/nrd4609. PMID 26091267. S2CID 25292436.
- ^ Yu H, Adedoyin A (Sep 2003). "ADME-Tox in drug discovery: integration of experimental and computational technologies". Drug Discovery Today. viii (18): 852–61. doi:ten.1016/S1359-6446(03)02828-9. PMID 12963322.
- ^ Dixon SJ, Stockwell BR (Dec 2009). "Identifying druggable illness-modifying gene products". Current Opinion in Chemical Biological science. 13 (v–6): 549–55. doi:ten.1016/j.cbpa.2009.08.003. PMC2787993. PMID 19740696.
- ^ Imming P, Sinning C, Meyer A (Oct 2006). "Drugs, their targets and the nature and number of drug targets". Nature Reviews. Drug Discovery. five (10): 821–34. doi:ten.1038/nrd2132. PMID 17016423. S2CID 8872470.
- ^ Anderson Air conditioning (Sep 2003). "The process of construction-based drug design". Chemistry & Biology. 10 (nine): 787–97. doi:10.1016/j.chembiol.2003.09.002. PMID 14522049.
- ^ Recanatini M, Bottegoni Grand, Cavalli A (Dec 2004). "In silico antitarget screening". Drug Discovery Today: Technologies. 1 (3): 209–15. doi:x.1016/j.ddtec.2004.x.004. PMID 24981487.
- ^ Wu-Pong S, Rojanasakul Y (2008). Biopharmaceutical drug pattern and development (2nd ed.). Totowa, NJ Humana Press: Humana Press. ISBN978-1-59745-532-9.
- ^ Scomparin A, Polyak D, Krivitsky A, Satchi-Fainaro R (Apr 2015). "Achieving successful delivery of oligonucleotides - From physico-chemical characterization to in vivo evaluation". Biotechnology Advances. 33 (6): 1294–309. doi:10.1016/j.biotechadv.2015.04.008. PMID 25916823.
- ^ Ganellin CR, Jefferis R, Roberts SM (2013). "The pocket-sized molecule drug discovery process — from target selection to candidate selection". Introduction to Biological and Small Molecule Drug Research and Evolution: theory and case studies. Elsevier. ISBN9780123971760.
- ^ a b c Yuan Y, Pei J, Lai L (December 2013). "Binding site detection and druggability prediction of protein targets for structure-based drug design". Electric current Pharmaceutical Pattern. xix (12): 2326–33. doi:ten.2174/1381612811319120019. PMID 23082974.
- ^ Rishton GM (Jan 2003). "Nonleadlikeness and leadlikeness in biochemical screening". Drug Discovery Today. 8 (2): 86–96. doi:10.1016/s1359644602025722. PMID 12565011.
- ^ Hopkins AL (2011). "Affiliate 25: Pharmacological space". In Wermuth CG (ed.). The Practice of Medicinal Chemistry (3 ed.). Bookish Printing. pp. 521–527. ISBN978-0-12-374194-3.
- ^ Kirchmair J (2014). Drug Metabolism Prediction. Wiley'due south Methods and Principles in Medicinal Chemistry. Vol. 63. Wiley-VCH. ISBN978-3-527-67301-8.
- ^ Nicolaou CA, Dark-brown North (Sep 2013). "Multi-objective optimization methods in drug design". Drug Discovery Today: Technologies. 10 (3): 427–35. doi:10.1016/j.ddtec.2013.02.001. PMID 24050140.
- ^ Ban TA (2006). "The part of serendipity in drug discovery". Dialogues in Clinical Neuroscience. 8 (3): 335–44. doi:10.31887/DCNS.2006.8.three/tban. PMC3181823. PMID 17117615.
- ^ Ethiraj SK, Levinthal D (Sep 2004). "Bounded Rationality and the Search for Organizational Architecture: An Evolutionary Perspective on the Design of Organizations and Their Evolvability". Administrative Science Quarterly. Sage Publications, Inc. on behalf of the Johnson Graduate Schoolhouse of Management, Cornell University. 49 (three): 404–437. doi:x.2307/4131441. JSTOR 4131441. S2CID 142910916. SSRN 604123.
- ^ Lewis RA (2011). "Chapter 4: The Development of Molecular Modelling Programs: The Use and Limitations of Physical Models". In Gramatica P, Livingstone DJ, Davis AM (eds.). Drug Design Strategies: Quantitative Approaches. RSC Drug Discovery. Royal Lodge of Chemistry. pp. 88–107. doi:ten.1039/9781849733410-00088. ISBN978-1849731669.
- ^ Rajamani R, Good Ac (May 2007). "Ranking poses in structure-based lead discovery and optimization: current trends in scoring part development". Electric current Stance in Drug Discovery & Development. ten (iii): 308–fifteen. PMID 17554857.
- ^ de Azevedo WF, Dias R (Dec 2008). "Computational methods for calculation of ligand-binding affinity". Current Drug Targets. 9 (12): 1031–9. doi:10.2174/138945008786949405. PMID 19128212.
- ^ Singh J, Chuaqui CE, Boriack-Sjodin PA, Lee WC, Pontz T, Corbley MJ, Cheung HK, Arduini RM, Mead JN, Newman MN, Papadatos JL, Bowes S, Josiah Southward, Ling LE (Dec 2003). "Successful shape-based virtual screening: the discovery of a potent inhibitor of the blazon I TGFbeta receptor kinase (TbetaRI)". Bioorganic & Medicinal Chemical science Letters. xiii (24): 4355–9. doi:10.1016/j.bmcl.2003.09.028. PMID 14643325.
- ^ Becker OM, Dhanoa DS, Marantz Y, Chen D, Shacham S, Cheruku Southward, Heifetz A, Mohanty P, Fichman M, Sharadendu A, Nudelman R, Kauffman M, Noiman S (Jun 2006). "An integrated in silico 3D model-driven discovery of a novel, potent, and selective amidosulfonamide 5-HT1A agonist (PRX-00023) for the handling of anxiety and depression". Journal of Medicinal Chemical science. 49 (11): 3116–35. doi:10.1021/jm0508641. PMID 16722631.
- ^ Liang S, Meroueh SO, Wang Yard, Qiu C, Zhou Y (May 2009). "Consensus scoring for enriching nearly-native structures from poly peptide-protein docking decoys". Proteins. 75 (two): 397–403. doi:10.1002/prot.22252. PMC2656599. PMID 18831053.
- ^ Oda A, Tsuchida One thousand, Takakura T, Yamaotsu N, Hirono Due south (2006). "Comparison of consensus scoring strategies for evaluating computational models of protein-ligand complexes". Journal of Chemic Data and Modeling. 46 (1): 380–91. doi:x.1021/ci050283k. PMID 16426072.
- ^ Deng Z, Chuaqui C, Singh J (Jan 2004). "Structural interaction fingerprint (SIFt): a novel method for analyzing three-dimensional protein-ligand binding interactions". Periodical of Medicinal Chemistry. 47 (two): 337–44. doi:10.1021/jm030331x. PMID 14711306.
- ^ Amari S, Aizawa M, Zhang J, Fukuzawa Yard, Mochizuki Y, Iwasawa Y, Nakata 1000, Chuman H, Nakano T (2006). "VISCANA: visualized cluster analysis of protein-ligand interaction based on the ab initio fragment molecular orbital method for virtual ligand screening". Journal of Chemic Data and Modeling. 46 (one): 221–30. doi:10.1021/ci050262q. PMID 16426058.
- ^ Guner OF (2000). Pharmacophore Perception, Evolution, and use in Drug Design. La Jolla, Calif: International University Line. ISBN978-0-9636817-6-8.
- ^ Tropsha A (2010). "QSAR in Drug Discovery". In Reynolds CH, Merz KM, Ringe D (eds.). Drug Design: Structure- and Ligand-Based Approaches (1st ed.). Cambridge, UK: Cambridge University Printing. pp. 151–164. ISBN978-0521887236.
- ^ Leach AR, Harren J (2007). Structure-based Drug Discovery. Berlin: Springer. ISBN978-i-4020-4406-9.
- ^ Mauser H, Guba West (May 2008). "Recent developments in de novo design and scaffold hopping". Current Opinion in Drug Discovery & Development. 11 (3): 365–74. PMID 18428090.
- ^ a b Klebe K (2000). "Contempo developments in structure-based drug design". Journal of Molecular Medicine. 78 (5): 269–81. doi:10.1007/s001090000084. PMID 10954199. S2CID 21314020.
- ^ Wang R, Gao Y, Lai 50 (2000). "LigBuilder: A Multi-Purpose Program for Structure-Based Drug Design". Journal of Molecular Modeling. 6 (7–8): 498–516. doi:ten.1007/s0089400060498. S2CID 59482623.
- ^ Schneider K, Fechner U (Aug 2005). "Computer-based de novo design of drug-like molecules". Nature Reviews. Drug Discovery. 4 (8): 649–63. doi:10.1038/nrd1799. PMID 16056391. S2CID 2549851.
- ^ Jorgensen WL (Mar 2004). "The many roles of computation in drug discovery". Science. 303 (5665): 1813–8. Bibcode:2004Sci...303.1813J. doi:10.1126/science.1096361. PMID 15031495. S2CID 1307935.
- ^ a b Leis S, Schneider S, Zacharias Chiliad (2010). "In silico prediction of binding sites on proteins". Current Medicinal Chemistry. 17 (15): 1550–62. doi:ten.2174/092986710790979944. PMID 20166931.
- ^ Warren GL, Warren SD (2011). "Chapter 16: Scoring Drug-Receptor Interactions". In Gramatica P, Livingstone DJ, Davis AM (eds.). Drug Design Strategies: Quantitative Approaches. Royal Society of Chemistry. pp. 440–457. doi:10.1039/9781849733410-00440. ISBN978-1849731669.
- ^ Böhm HJ (Jun 1994). "The evolution of a unproblematic empirical scoring function to approximate the binding constant for a poly peptide-ligand complex of known three-dimensional structure". Journal of Computer-Aided Molecular Pattern. eight (3): 243–56. Bibcode:1994JCAMD...8..243B. doi:10.1007/BF00126743. PMID 7964925. S2CID 2491616.
- ^ Liu J, Wang R (23 March 2015). "Classification of Electric current Scoring Functions". Journal of Chemical Information and Modeling. 55 (3): 475–482. doi:10.1021/ci500731a. PMID 25647463.
- ^ Ajay, Murcko MA (1995). "Computational methods to predict binding energy in ligand-receptor complexes". J. Med. Chem. 38 (26): 4953–67. doi:10.1021/jm00026a001. PMID 8544170.
- ^ Gramatica P (2011). "Affiliate 17: Modeling Chemicals in the Surround". In Gramatica P, Livingstone DJ, Davis AM (eds.). Drug Design Strategies: Quantitative Approaches. RSC Drug Discovery. Royal Society of Chemistry. p. 466. doi:10.1039/9781849733410-00458. ISBN978-1849731669.
- ^ Greer J, Erickson JW, Baldwin JJ, Varney MD (April 1994). "Application of the three-dimensional structures of poly peptide target molecules in structure-based drug design". Journal of Medicinal Chemistry. 37 (viii): 1035–54. doi:ten.1021/jm00034a001. PMID 8164249.
- ^ Timmerman H, Gubernator One thousand, Böhm HJ, Mannhold R, Kubinyi H (1998). Structure-based Ligand Design (Methods and Principles in Medicinal Chemistry). Weinheim: Wiley-VCH. ISBN978-3-527-29343-8.
- ^ Capdeville R, Buchdunger Eastward, Zimmermann J, Thing A (Jul 2002). "Glivec (STI571, imatinib), a rationally adult, targeted anticancer drug". Nature Reviews. Drug Discovery. 1 (seven): 493–502. doi:10.1038/nrd839. PMID 12120256. S2CID 2728341.
- ^ "AutoDock's role in Developing the First Clinically-Canonical HIV Integrase Inhibitor". Press Release. The Scripps Research Plant. 2007-12-17.
- ^ Klein DF (Mar 2008). "The loss of serendipity in psychopharmacology". JAMA. 299 (9): 1063–v. doi:ten.1001/jama.299.9.1063. PMID 18319418.
External links [edit]
- Drug+Design at the U.s. National Library of Medicine Medical Subject field Headings (MeSH)

![{\displaystyle {\begin{array}{lll}\Delta G_{\text{bind}}=-RT\ln K_{\text{d}}\\[1.3ex]K_{\text{d}}={\dfrac {[{\text{Ligand}}][{\text{Receptor}}]}{[{\text{Complex}}]}}\\[1.3ex]\Delta G_{\text{bind}}=\Delta G_{\text{desolvation}}+\Delta G_{\text{motion}}+\Delta G_{\text{configuration}}+\Delta G_{\text{interaction}}\end{array}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ba49ddd9dec7415d129787213744ca1afcd2d021)
0 Response to "Plus Sized Fashion Design Model Sketch"
Post a Comment